
Biochemistry of a burger
In what has become a journalistic cliché, I took my boyfriend, a vegetarian for the past 10 years, to our local greasy spoon to try out an Impossible Burger.
He didn’t care for it.
He had lost his taste for ground beef and found the experience of a plant-based, completely vegan yet beefy-tasting burger less than appealing. I, on the other hand, was a fan; his burger tasted better to me than my own, which was made the old-fashioned way, with beef.
People like me are exactly the people Impossible Burger is targeting: millennials who eat meat sometimes but fret about its impacts on a crowded, warming world.
David Welch is the director of science and technology at the Good Food Institute, a think tank promoting the burgeoning plant-based meat industry. He said one of the reasons Impossible Foods and its strongest competitor, Beyond Meat, have garnered so much interest from investors is their average customer.
“They’re not targeting the vegan or vegetarian consumer,” Welch said. “They’re targeting the meat eater who is being more conscious about their decision.”

“We were approaching (meat) the same way you might approach figuring out the entire system for replicating genes,” Brown said during an interview in June. “Basically, start looking at what are all the possible components that could be players … and then deconstruct and reconstitute the biochemical system.”
Market and mission
The world eats a lot of beef: 57 pounds per person per year in the U.S., with consumption . Because cattle are the livestock animal at converting feed into edible meat, environmentalists have argued for years that reducing beef consumption is an important conservation act.
Brown, who has been vegetarian for decades, says that those arguments have not been effective, even when made by parties as influential as the . “That experiment has been done,” he told an audience of microbiologists during the American Society for Microbiology’s in June, which he delivered wearing a “no cows” T-shirt. “You don’t solve the problem by asking people to change their diets.”
Brown frames the problem instead as relying on an outdated technology — namely the cow — for beef production. He wants to solve the problem by making meat imitations so appealing that they’ll outcompete what he calls the legacy industry. Impossible Foods explicitly states that its mission is to eliminate all animals from the food production system by 2035.
Staffing with scientists
Becoming a CEO wasn’t in Brown’s original plan. Compared with being a professor, he thought a job in business would be dull. He first set out to eradicate animal agriculture while on sabbatical from Stanford in 2009 and thought he could do it by persuading someone else to take on disrupting the industry.
He anticipated that an established food producer would adopt the meat-alternative idea as enthusiastically as he had. “I went around to food industry conferences and gave a spiel to the effect that someone’s going to make a killing if they are the first mover to replace animals in food production technology,” he said in 2017. “That was stupid.”
When the food industry didn’t bite and a workshop he organized at the National Academies resulted in no discernible policy change, Brown decided to do it himself.
“I was not a savvy business person, but I had the very good fortune of living in the epicenter of venture capital,” he said. The funding may have had less to do with Brown’s physical proximity to the venture capital firms of San Francisco than his track record of disrupting scientific fields.
Early on, Brown hired mostly biochemists, many from his professional circles at Stanford — along with some of his closest relatives. “I knew a lot of talented students and postdocs, and a lot of my friends were scientists and they knew a lot of people,” he said. “Of course, there was no field of creating meat directly from plants, so they came from all kinds of backgrounds. All I really wanted was raw scientific talent.”
According to the David Welch, staffing at Impossible Foods is unlike that at more established meat-alternative companies.

“At tech-focused companies like Impossible Foods and Beyond Meat, the R&D teams are full of biochemists,” he said. “A more traditional plant-based meat company may have had a different balance in terms of the scientific talent within that company, with much more focus on food science and culinary aspects of assembling ingredients.”
Brown said that he looked for scientists who were up for a challenge. “I had the most awesome recruiting tool, which is that I could tell them with a straight face that we are working on unequivocally the most important scientific problem in the world, and it’s really challenging. If you want to get great scientists to work for you, that’s pretty much what you need.”
For people like , a senior flavor scientist at Impossible Foods, the company’s environmental mission was a selling point. In 2016, Kliman, an organic chemist by training, was working as a pastry chef. She had just left a job as a chemist at a company that once developed biofuels but was transitioning to natural gas. She heard about Impossible Foods from a news story on the radio.
“I couldn’t believe how exciting and perfect it was,” she said. “Obviously extremely focused on sustainability, while incorporating culinary (aspects) as well as chemistry.”
, head of the department of food science at the University of Massachusetts at Amherst, said that Impossible Foods is not unique in hiring scientists with no food science training.

“There have been biochemists — and organic chemists, and analytical chemists — in the food sector for decades,” Decker said. “And flavor houses have been trying to replicate meat for a long time.”
Rather, he said, what sets Impossible Foods apart are its startup-style business model, which has allowed the company to invest tremendous resources in a single product, and its patented plant-based heme protein ingredients.
Flavor chemistry
The team at Impossible Foods set out to define a burger biochemically by heating grocery store ground beef and, with a gas chromatography system and a mass spectrometer, characterizing the products of the complex reactions that resulted as it cooked. Then, instead of trying to incorporate all the aromatic chemicals they identified into a veggie patty, Brown’s team used precursors to the final flavor compounds, letting the chemistry happen during cooking.
“One of the things that I think sets us apart,” Kliman said, “is that we really took the time to research meat on the molecular level and understand all the actual reactions that are happening and what the products are that are being created.”
According to most coverage of Impossible Foods, its scientists were the very first to take a reductionist approach to replicating the flavor of meat. The scientific literature tells a more complex story.
An industry report for the National Cattlemen’s Beef Association in 2006 spanning decades of analytical chemistry work in numerous food science journals, most of which aimed to identify the most important flavor compounds in beef and determine what affected them; Brown and colleagues had a lot of background to work with. That report focused on optimizing husbandry, meat handling and processing practices to improve the final flavor.
But in other contexts, scientists have worked for years to develop additives based on a thorough understanding of meat chemistry. As early as the 1960s, food companies like Procter and Gamble that could give bland protein isolates a beefy flavor upon heating.
Chemists working to recreate beef flavors on two major types of reactions that occur when a piece of meat hits a hot grill: lipid oxidation and Maillard reactions.

occurs when reactive oxygen species oxidize lipids, especially polyunsaturated fatty acids, at their double bonds. The reaction can generate low–molecular-weight fission products and, sometimes, short-lived epoxide compounds; some of these taste appealing, but most do not.
“Iron is the major problem in any (food) that undergoes oxidation,” Decker said. Either free or balanced in the aromatic, heterocyclic ring complex of heme, iron can catalyze oxidation of polyunsaturated fatty acids, resulting in a warmed-over flavor.
, the second major reaction family driving beef flavor, happen when an amino acid and a sugar are mixed and heated. The reaction, an umbrella term for condensation between a nucleophilic amine and an electrophilic carbonyl, is important in cooking chemistry from browning pretzels to flavoring roasts. One of the best-studied Maillard reactions involved in flavoring meat takes place when ribose and cysteine are heated, creating a range of cyclic volatile compounds that contribute characteristic flavor notes to meat. Hydrogen sulfide derived from cysteine plays an important role in making some of those compounds, catalyzing addition of sulfhydryl groups to reaction products.
Inorganic iron to speed up Maillard reactions. But a key part of Impossible Foods’ story is its discovery of a new effect of heme iron, which the company calls its “magic ingredient,” on the flavor of meat.
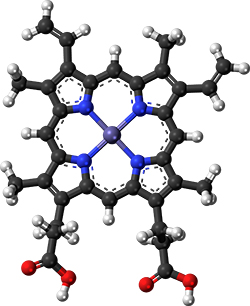
The magic molecule
“How does it taste?” Brown said. “Basically, that’s just an assay — like radioactive thymidine incorporation in the early days of DNA replication. It’s just an assay that tells you whether what you’re trying to get to happen is actually happening. And then, you deconstruct and reconstitute the biochemical system.”
If a researcher heats a simple solution of cysteine and glucose, asserts, the product will have a faint rotten-egg smell. Should that researcher add ferrous gluconate, which contains an iron cation similar to the one in heme, again the product smells sulfurous. But replace the gluconate with a heme protein, and that unappetizing odor is replaced with smells that evoke chicken broth, burnt mushrooms and bread.
Biochemically, what’s so special about heme? Unsurprisingly, Impossible Foods has not volunteered chemical specifics. Given the odors mentioned in the patent, it’s tempting to speculate that the answer may have something to do with interaction between heme and hydrogen sulfide derived from cysteine.

According to , whose lab at the University of Michigan Medical School studies hydrogen sulfide signaling, such speculation is not unreasonable. that hemoglobin in red blood cells, which lack normal hydrogen sulfide turnover pathways, can oxidize hydrogen sulfide to generate more complex sulfur-containing molecules.
“The biochemical evidence for this chemistry is very strong,” Banerjee said. “It’s not just reversible binding; the sulfide is actually transformed by the heme in air to oxidized products.”
Perhaps such catalysis is part of how heme diminishes the off-putting odors and promotes more palatable flavors in the simple two-ingredient mixture. By adding additional molecules — ribose, thiamine, various amino acids and lipids — the researcher can make the product both more complex and more reminiscent of meat.

This, in essence, is the approach the Impossible Foods team took. A patent describes a mixture of amino acids, sugars and metabolites titrated to match meat tissue; when heated with leghemoglobin, it enables the key reactions that build a meat flavor profile. Meanwhile, according to Decker, levels of polyunsaturated fats are lower in the Impossible Burger than in meat, which may prevent the added heme from boosting unpleasant flavors.
But applying insights from a simple mixture of two or three ingredients to the complex chemical and material mixture in a vegetable patty is a difficult task.
“As an organic chemist, you have so much control over what’s in your reaction. And that’s just not the case with food,” Kliman said. “There are so many reactions that it’s hard to track them all and to know what products are being produced from what sort of precursors.”
Globins without animals
When characterizing meat, Brown said, “The thesis was that there’s absolutely nothing that was required that was specific to animals. That could have been wrong. It turned out to be right, which was good.”
Brown thought he knew a good place to harvest heme without killing an animal: the root nodules of legumes. , a colleague on the biochemistry faculty at Stanford, studied these tiny hotbeds of nitrogen fixation, and Brown was familiar with their iron-rich microecosystem.
“We spent quite a lot of money and quite a large fraction of our time in the first year and a half on this quest to figure out a way to harvest root nodules … and creating all these kind of Rube Goldberg contraptions,” Brown said with the American Society for Microbiology’s Julie Wolf.
It turned out that harvesting and cleaning root nodules and extracting their leghemoglobin, while possible, was impractical at an industrial scale. Instead, the team pivoted to producing heme-binding proteins in yeast.

Constant optimization
Though heme as a magic molecule is key to the company’s brand narrative, Brown often stresses that there’s more to an Impossible Burger than that.
“If you’ve nailed the flavor chemistry and nothing else, well, you have beef broth,” he said. “And if you’ve nailed the texture, and you don’t have the flavor, you have crab cakes. … Basically, the more progress we make on one dimension, the more we think, ‘Oh, we’ve gotta make more progress on the next.’”
The first prototype ranked somewhere below crab cakes or beef broth. Supertasters on staff compared it to “rancid polenta.” But, according to Brown, improvement was swift after that. This is part of the company’s mindset; a perfect reproduction of ground beef is not its end goal. If chemists can improve on the eating experience, producing something with fewer off flavors or a more palatable texture, then they will.
“In a certain sense, we’ll declare victory when the incumbent industry is in the rearview mirror,” Brown said. “But, on the other hand, to the extent that we can make people happier and our product more affordable and versatile and food secure, we’re going to just keep working and working. I’m not sure whether it will ever end.”
Production problems
This year, Impossible Foods introduced a new formulation that chefs say is a better match for beef, but the company was also hindered by its own runaway success. After launching the new recipe, this spring the company became unable to keep up with accelerating demand for its products. The timing was unfortunate; the company was in the midst of a regional pilot partnership with Burger King.

Brown that the company’s efforts to scale up production had been “like changing the tires while driving down the freeway.”
Talking to 91—«…´¥´√Ω Today, he elaborated on their reaction to the shortage. “When we realized that manufacturing was limiting, I sent out a call to everybody in our R&D facility operation, basically saying, ‘OK, I need volunteers to work 12-hour shifts that either start or end at 3 a.m., just doing whatever it takes in a room that’s 37 Fahrenheit. And it’s across the (San Francisco) Bay.’ And within 24 hours, a hundred people had volunteered.”
He tells the story with evident pride in his team’s dedication to the mission. On the , where workers can post reviews of their employers, anonymous posts reveal mixed feelings about the company’s monthslong production sprint, and several note a lack of food industry know-how.
Others, however, like the challenge. Kliman, the flavor scientist, said, “In an academic lab — this is sort of a sweeping generalization — you have a lot of time, and you have almost no money. So you get creative about ways to solve problems. In an industry (lab), let’s say pharma, it’s very much about the bottom line, and time is money. Here at Impossible, it’s like the best of both worlds,” with a dual focus on optimizing the company’s products and contributing ideas for new ones. She cited periodic innovation weeks when the research and development team spends all its time trying new ideas or experiments.
In April, Impossible Foods hired Dennis Woodside, a lawyer by training with some 16 years of corporate leadership experience, into the newly created role of president. Having a president, Brown said in June, would free him up to focus on future innovations — as soon as the company caught up to its manufacturing shortfall. “I want to spend a lot more time on R&D, but even our R&D team are all hands on deck trying to get this manufacturing caught up,” he said.
In July, the company declared an end to the shortage and announced its plans to open a in Chicago.
Changing the meat industry
For the Impossible Foods plan to succeed, ranching needs to go from marginally profitable to losing money. This is not at all popular in the beef industry, which for restrictions on marketing plant-based foods as meat. The product, on the other hand, is growing in popularity — and improving as a substitute.
According to , in 2019 plant-based meat is a $12 billion industry, and it’s expected to grow. Impossible Foods, which is privately owned, does not report its sales figures. But its closest competitor, Beyond Meat, reported that its revenues from 2017 to 2018.
In light of that reality, more of the food industry is beginning to adopt plant-based protein production. This year, Tyson Foods, the largest meat company in the United States, appointed a vice president for alternative proteins and sustainability and announced a new brand that’s set to launch this fall. Even legacy companies like Morningstar Farms, which has been making plant-based protein foods for 40 years, have introduced new faux meats that seek to compete more successfully against Impossible Foods and Beyond Meat.
Somewhat upstream from burger production, ingredient companies are aiming to catch the plant-based meat wave by providing pure protein and other ingredients. For example, is a Boston startup that focuses on using biotechnology methods to produce components other food companies can use.
“Motif is somewhat unique in that they’re taking a synthetic biology or recombinant biology approach to creating new ingredients,” Welch said. The approach is similar to the way Impossible Foods generated a yeast strain to produce soy leghemoglobin at scale. Welch said that Motif is trying to do similar things with other possible ingredients, such as milk proteins.
There’s also some evidence that the surge in popularity of plant-based meat is changing American crop fields. “We’re starting to see some examples of it now,” Welch said. “I think, in the future, we’ll see more examples of crops being grown that are specifically bred for plant-based meat applications.”
Protein foods for the future
With characteristic confidence, Brown has moved on to making plans to further redefine the food industry of the future.
“If the technology we develop is used to produce a significant fraction of the world’s food supply and protein and so forth, it’s a huge responsibility,” he said in June. “Impossible Foods the company is not going to be responsible for all of that — but we’re developing the technology to enable (it).”
The company has announced that prototypes of chicken, fish and even eggs, all made without animal products, are in hand. Meanwhile, a crop of smaller competitors also are developing and introducing their replicas of those foods.

Now, Brown is thinking about what plant ingredients to use as source material. One possibility that seems to have caught his imagination — lately he has volunteered it a lot in interviews — is to replace plant-derived protein from grains and legumes with protein from leaves. Impossible Foods has patented an approach to purify the protein rubisco in bulk from the leavealfs of alfa, a crop presently used for animal fodder, or other plants. But according to David Welch, the protein feedstocks of the future are as likely to be generated from bacteria, algae or fungi as from traditional crops.
“We’re constantly screening things,” Kliman said. “Anything you can think of to generate a plant-based protein, or other sorts of nutrients and ingredients, we’re interested in looking at that and seeing if we can turn it into something amazing.”
“People are always telling me, ‘You’ve got to focus more,’” Brown said. “And I feel like being unfocused is incredibly valuable … when you know where you want to go, but there’s no road map, and you have to figure out how to get there.
“The last thing you want to do is say, ‘I’m just going to go down this trail and I’m gonna keep going until I fall off a cliff.’ No. I’m going to explore and wander.”
And as a postscript: This fall, my vegetarian boyfriend gave the Impossible Whopper a try. He enjoyed it enough to order a second — Impossible Foods converts another customer.
A research career
Before launching Impossible Foods, Pat Brown was already well known for a 30-year career in research and scientific activism. Now, he’s so famous as an entrepreneur that news articles sometimes mention that first life — during which he established the field of functional genomics and started rolling a rock that’s now an avalanche of open-access publication in the life sciences — as an afterthought.
According to his colleagues, Brown always has excelled at coming up with creative solutions to difficult problems. Throughout his career, these problems have grown in scale and expanded in impact beyond the lab bench.
Brown learned the process of detailed biochemical investigation, which his team would later apply to the humble hamburger, from some of the most noted biochemists of the 20th century. As a graduate student at the University of Chicago, he trained with the late . After finishing a Ph.D. and a medical residency, Brown joined the lab of biochemist J. Michael Bishop at the University of California, San Francisco, as a postdoc, where he collaborated closely with members of ’ lab to study HIV molecular biology.
, who worked closely with Brown at UCSF, recollected a sign over Brown’s lab bench “that said, in bold black letters, ‘If I have seen farther, it is by standing on the shoulders of —’ and instead of ‘giants,’ which is the Newton quote, he had written, ‘idiots.’
“Pat was not a modest person,” he said. “But he wasn’t unpleasant at all.”
In 1988, Brown was hired as an assistant professor at Stanford, where he would remain on the faculty for 25 years. The scientific work he’s most famous for in that time is developing the , a technique that originally was envisioned for genetic mapping but became popular as the first method to measure changes to a whole transcriptome.
To interpret the data on regulation of thousands of genes produced by a microarray, Brown and his team hoped to mine the scientific literature, which was beginning to be available online. But they found that journals, which depended on subscriptions, weren’t open to the idea. With Varmus and Michael Eisen, a postdoc in his lab, Brown embarked on an advocacy project for open access to the scientific literature that started with a proposal to expand PubMed and culminated in founding a new publisher, the .
When Brown decided to run Impossible Foods full time, he left Stanford, giving up his status as a Howard Hughes Medical Institute investigator. , a Nobel laureate who advised Brown as a postdoc, attended the HHMI meeting where Brown gave notice of his decision.
“Instead of giving a valedictory with his science, he gave a 20-minute talk on the ecological problem that he wanted to address,” Bishop said. It wasn’t a swan song; it was a presentation on his next big project.
“I think we were all surprised that someone as well situated as he was academically … and who still had a passion for the science would make that change,” Varmus said. “But I’d say it worked out pretty damn well for him.”

All in the family
There’s plenty of science in Impossible Foods founder Pat Brown’s family; his wife, Sue Klapholz, whom he met at the University of Chicago, holds an M.D. and a Ph.D. and works as the company’s vice president of nutrition and health. One of Brown’s brothers, a neuroscientist, has worked at the company for about three years as a sensory perception expert. But the most startling team member is Brown’s daughter, Ariel Klapholz–Brown, who studied film in college before starting as an early employee in the labs of the company that would become Impossible Foods. Today she’s a senior research associate on Impossible Foods’ materials and texture team.
“I love working with my daughter,” Brown said, adding that she’d always been a talented math and science student. “She notices everything, which is just a great quality for the role that she’s in. There’s an art to creating new prototypes, and also there’s an incredibly important demand on observation. You have to pick up subtle things that you’re not looking for.”
On working with family and friends, he added, “If you have people that you know are all-in on what you’re doing and talented, and you know for sure that you can tolerate them, that they’re going to be good colleagues, then that’s great.”

Buffering oxygen

Legumes — plants like soy, pea and clover — are well known for converting atmospheric nitrogen into ammonia, which is a source of nutritional nitrogen. The reducing reaction called nitrogen fixation, for which the plants often get credit, is in fact carried out by symbiotic bacteria that live in specialized organs in the plants’ roots. , chair of the biochemistry department at the University at Buffalo, studies nitrogen fixation by Bradyrhizobium japonicum, which colonizes soy roots.
A Bradyrhizobium enzyme called nitrogenase breaks the bonds in atmospheric nitrogen, O’Brian explained. The process demands a tremendous amount of energy, which Bradyrhizobium supplies through aerobic respiration. But an appetite for oxygen, the ultimate oxidizer, is perilous for the nitrogenase. Plant tissue in root nodules express a high level of leghemoglobin, a protein that, like other heme proteins, coordinates oxygen cooperatively.
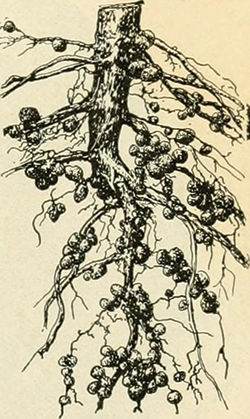
“You need oxygen for the bacteria to respire, but you can’t have a lot of free oxygen because it’s going to kill the nitrogenase. Leghemoglobin really resolves that paradox,” O’Brian said, by binding with high affinity to free oxygen and releasing it only when the concentration of oxygen is low. “Essentially, it’s facilitating oxygen diffusion, which is similar to what myoglobin does in the muscle of animals.”
Since Impossible Foods has become prominent, O’Brian said, he has found it easier to to nonscientists.
“If you say, ‘We work on ABCD,’ it’ll put people to sleep. But if you say, ‘This is being used for the Impossible Burger,’ people are interested.”

Enjoy reading 91—«…´¥´√Ω Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from 91—«…´¥´√Ω Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Industry
Industry highlights or most popular articles

Black excellence in biotech: Shaping the future of an industry
This Black History Month, we highlight the impact of DEI initiatives, trailblazing scientists and industry leaders working to create a more inclusive and scientific community. Discover how you can be part of the movement.

Attend 91—«…´¥´√Ω‚Äôs career and education fair
Attending the 91—«…´¥´√Ω career and education fair is a great way to explore new opportunities, make valuable connections and gain insights into potential career paths.

Benefits of attending a large scientific conference
Researchers have a lot of choices when it comes to conferences and symposia. A large conference like the 91—«…´¥´√Ω Annual Meeting offers myriad opportunities, such as poster sessions, top research talks, social events, workshops, vendor booths and more.

Biotech startup worms its way into therapeutics
Andrea Choe's company, Holoclara, has created an anti-inflammatory drug based on a molecule from worms.

How military forensic scientists use DNA to solve mysteries
Learn how two analysts at the Armed Forces DNA Identification Laboratory use molecular biology and genetics to identify the remains of fallen troops.

Careers in industry: A year in review
Careers columnist Inayah Entzminger looks back at 12 months of interviews, advice and lists of resources.




