Oxygen sensing and adapting to altitude
All cells of the human body, even cancerous ones, live or die by their access to oxygen.
When that gaseeous molecule is in short supply, the body responds by upregulating the hormone erythropoietin, or EPO, to pump out more red blood cells, as well as proteins that affect wound healing, metabolism, embryonic development and altitude adjustment. This response also plays a role in anemia, stroke, infection, myocardial infarction and cancer.
This year, the Nobel committee awarded its prize for physiology or medicine to a trio of physician-scientists — at Johns Hopkins University, at the Dana-Farber Cancer Institute and at Oxford University — for their roles in discovering how cells sense and adapt to oxygen availability.
 Gregg L. Semenza decided to pursue genetics while at Harvard University when a family friend had a child born with Down syndrome.Johns Hopkins Medicine
Gregg L. Semenza decided to pursue genetics while at Harvard University when a family friend had a child born with Down syndrome.Johns Hopkins Medicine
In , Semenza characterized the protein complex hypoxia-inducible factor, or HIF, which controls the production of EPO and other proteins made in response to hypoxia, or reduced oxygen levels. In 1995, he the genes that encode the two subunits of HIF. During that time, his lab and Ratcliffe’s lab independently found that the oxygen-sensing mechanism is present in all bodily tissues rather than only in the kidney, where EPO is produced. Kaelin and his lab then found that the protein VHL, named after the inherited syndrome von Hippel-Lindau’s disease, was involved in controlling responses to hypoxia. Ratcliffe’s group subsequently found that VHL interacts with one of the two subunits of HIF, HIF-1 alpha, and tags it with ubiquitin for degradation at normal oxygen levels.
Semenza and his lab since have probed the role that HIF-1 and a related protein, HIF-2, play in sites across the body, including the breast cancer tumor microenvironment. He also has taken an interest in how variants in HIF pathway proteins have been selected over millennia in populations in Tibet that have adapted to living at high altitudes.
“We started with a really specific goal,” Semenza said. “We wanted to understand expression of one gene and how that gene responded to changes in oxygen in particular cells. And then, as we went on, the role of the HIFs just continued to expand and expand.”
Like many laureates before him, Semenza missed the first early-morning phone call from the Nobel committee in October.
“I was so sound asleep that, by the time I got to the phone, it had stopped ringing. So I went back to sleep,” he said. “It was actually quite some time later that the second call came and I thought ‘I better be quicker this time.’”
The news was an exceptionally welcome bright spot in 2019. “It was a pretty bad year up until a few weeks ago,” Semenza said during a mid-October interview in his lab at Hopkins.
In May, he fell down a flight of stairs and broke his neck. Hopkins was, by his account, a good place to be for that injury, from which he has recovered and has few complications. “Fortunately, we have an excellent neurosurgery department,” he said.
Winning the Nobel was exciting, but for Semenza, ever since he decided as an undergrad to study genetics, the focus always has been on how his research might affect patients’ well-being.
“One of the benefits of being trained as a physician is that that also gives you a certain perspective … and you see what patients and their families go through,” Semenza said. “Then an experiment that doesn’t work is not such an end-of-the-world thing.”
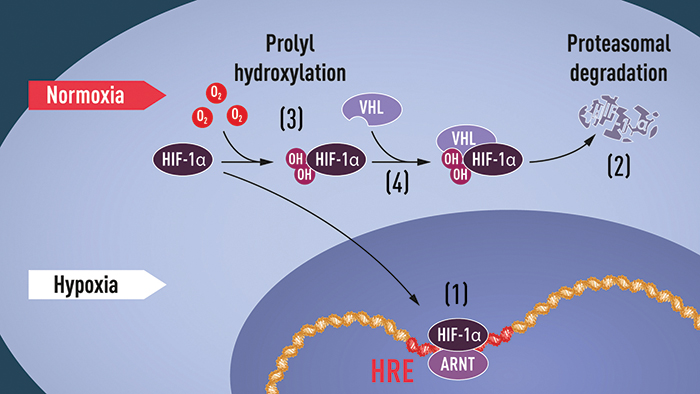
Semenza and his wife waited a few hours to tell their adult children about the Nobel committee’s call. In the meantime, he headed to his lab for an early-morning champagne toast.When oxygen levels are low (hypoxia), HIF-1 alpha is protected from degradation and accumulates in the nucleus, where it associates with ARNT and binds to specific DNA sequences (HRE) in hypoxia-regulated genes (1). At normal oxygen levels, HIF-1 alpha is degraded rapidly by the proteasome (2). Oxygen regulates the degradation process by the addition of hydroxyl groups (OH) to HIF-1 alpha (3). The VHL protein then can recognize and form a complex with HIF-1 alpha, leading to its degradation in an oxygen-dependent manner (4).
COURTESY KAROLINSKA INSTITUTE
Homing in on HIF
After graduating from Harvard University in 1978, Semenza enrolled in an M.D./Ph.D. program at the University of Pennsylvania. When he arrived at Hopkins in 1986 for a postdoctoral fellowship — fresh off a pediatrics residency at Duke University Medical Center — he intended to continue studying his thesis topic, the molecular basis of the hemoglobin deficiency disorder beta-thalassemia in transgenic mice. However, Semenza’s mentor at Hopkins, Haig Kazazian, and his colleague Stylianos Antonarakis had shifted to studying mutations in the factor VIII gene, which is essential to blood clotting.
Finding EPO
The French physician Paul Carnot and his graduate student Clotilde-Camille Deflandre proposed hormonal regulation of red blood cell production in after investigating the effects of bloodletting on red blood cell concentration in rabbits. In 1948, Finnish scientists Eva Bonsdorff and Eeva Jalavisto named the hormone erythropoietin for the erythrocyte blood precursors found in bone marrow.
Eugene Goldwasser’s lab at the University of Chicago purified EPO in 1977. In collaboration with the biopharmaceutical company anemia in people with kidney disease. The U.S. Food and Drug Administration approved the drug, sold as Epogen, in 1989. Amgen, Goldwasser then isolated the EPO gene and expressed it in an epithelial cell line. This paved the way for the drug epoetin alfa, developed to treat anemia in people with kidney disease. The U.S. Food and Drug Administration approved the drug, sold as Epogen, in 1989.
Semenza decided to focus on EPO expression (see box: Finding EPO); at the time, the hormone was believed to be expressed in the liver during early development but only in the kidney during adulthood.
The research team took advantage of the EPO gene’s small size by inserting a fragment containing the whole gene and small flanking sequences, for a total of about 4 kilobases of DNA, into transgenic mice. As hoped for, this yielded expression of EPO in the liver of adult specimens.
Due to the presence of both the human and mice EPO genes, the mice produced too many red blood cells, a condition called polycythemia.
“But the gene was not expressed in a regulated way in the kidney,” Semenza said. “And it was expressed in a lot of places where we thought it shouldn’t be expressed, including the brain.”
The researchers then inserted a larger piece of DNA, which curtailed some of the extraneous EPO expression. When they inserted an even larger fragment, which included important flanking sequences, they found that the protein was expressed exclusively in the fibroblast kidney cells where endogenous EPO is expressed.
“It was only then, after we had done all those experiments, that I began to think about the mechanisms for regulating the gene according to oxygen tension,” Semenza said.
The researchers soon found that a short DNA sequence , which they named the hypoxia-response element, controlled the increase of EPO gene transcription in response to hypoxia. Over the following years, Semenza and his postdoc Guang Wang identified, characterized and purified a protein that bound to the HRE, which Semenza named HIF-1, and identified of its two subunits, designated as HIF-1 alpha and HIF-1 beta.
At low oxygen levels, HIF-1 alpha accumulates in the nucleus, where it and HIF-1 beta, which is identical to the protein ARNT, bind to and upregulate the EPO gene, stimulating production of hemoglobin. However, at normal oxygen levels, HIF-1 alpha rapidly is degraded by proteasomes, which target the protein once it has been tagged with ubiquitin by the von Hippel-Lindau protein.
Kaelin, Ratcliffe and VHL
William Kaelin, who shared the Nobel Prize with Gregg Semenza and Peter Ratcliffe, discovered the von Hippel-Lindau, or VHL, protein — named for a genetic disease — when he was investigating its effects in families who had inherited a mutated form of it and, as a consequence, suffered dramatically increased risk of certain cancers. He found that cancer cells that did not express VHL expressed high levels of genes normally regulated by hypoxia, such as those related to angiogenesis, and thus tumor growth. However, the oxygen-dependent mechanisms that told VHL when to tag HIF-1 alpha with ubiquitin remained a mystery until , when Kaelin and Ratcliffe found that, at normal oxygen levels, oxygen-sensitive enzymes called prolyl-hydroxylases modify HIF-1 alpha with hydroxyl groups that interact with VHL, bringing the entire mechanism into focus.
Adaptations to altitude
Since puzzling out how HIF allows cells to sense oxygen levels, Semenza has wanted to know how that system adapts to the reduced oxygen in high-altitude environments such as Tibet and the Bolivian Andes. When someone whose family has lived for generations at low elevation rapidly ascends to altitudes above 8,000 feet, they can develop symptoms of acute mountain sickness, such as headaches, dizziness, nausea and exhaustion. Over time, this can develop into chronic mountain sickness, which is characterized by polycythemia and increased blood pressure; in severe cases, this becomes high-altitude pulmonary edema.
“As the red blood cell count goes up, the blood becomes more viscous and may be more likely to cause complications, particularly in pregnancy,” Semenza said.
“When you think about it, the problem is not too few red blood cells, it’s too little oxygen.”
In , Semenza and his collaborator sequenced the HIF-containing genomic regions of 26 people from Tibet. To the researchers’ surprise, they found that hemoglobin levels don’t spike in the Tibetans and that a mutation was responsible for a lower oxygen threshold for HIF to signal EPO production.
“It was basically gain of function in Tibetans of negative regulators of HIFs,” Prchal said. “So in hypoxia, the Tibetans downregulated HIF.”
 Gregg L. Semenza, left, and Josef Prchal, right, have been collaborators for over 30 years. In 2017, they visited a Mongolian nomad settlement on the Tibetan Plateau in China’s Qinghai Province with Ge Ri-Li, director of the Research Center for High Altitude Medicine at Quighai University, and high-altitude anthropologist Cynthia M. Beall. JOSEF PRCHAL
Gregg L. Semenza, left, and Josef Prchal, right, have been collaborators for over 30 years. In 2017, they visited a Mongolian nomad settlement on the Tibetan Plateau in China’s Qinghai Province with Ge Ri-Li, director of the Research Center for High Altitude Medicine at Quighai University, and high-altitude anthropologist Cynthia M. Beall. JOSEF PRCHAL
According to Semenza, Prchal was interested in finding mutated HIF variants after noticing that previous studies of people in Tibet were all missing a specific sequence.
“He found that that was very difficult to sequence, so he developed a protocol for getting it,” Semenza said. “And lo and behold, there was a polymorphism there that affected the amino acids. And remarkably, it occurred on the background of a gene that already had a variant at another site in the protein.”
Were it not for that first mutation, the second one, which was associated with low levels of hemoglobin, would have been impossible to develop.
“He calculated that this second mutation occurred 8,000 years ago on the background of this C-SNP (cysteine single nucleotide polymorphism),” Semenza said.
In a , Prchal and colleagues at the University of Copenhagen found that a similar mutation had evolved in populations in the Bolivian Andes.
“Nobody went from Tibet to the Andes and said, ‘I’ve got a few variants you might need,’ right? This occurred independently,” Semenza said.
Independent groups also have found that HIF-2 alpha is mutated in both and .
“This is how you get to the best state under high altitude, right?” Semenza said. “You make a variant right in this gene … medicine, biology, evolution — it’s amazing.”
Partners and mentors
Prchal, a hematologist at the University of Utah and native of the Czech Republic, has been collaborating with Semenza since the latter was a postdoc at Hopkins.
“He’s an extremely focused and driven person,” Prchal said. “If I need any advice about some very difficult biological puzzle, I don’t think anybody can give me better advice than Gregg.”
 Daniele Gilkes’ research at Johns Hopkins University focuses on the genetic alterations that happen under hypoxic conditions and how they can both contribute to tumor progression and be prevented.Courtesy of Daniele Gilkes
Daniele Gilkes’ research at Johns Hopkins University focuses on the genetic alterations that happen under hypoxic conditions and how they can both contribute to tumor progression and be prevented.Courtesy of Daniele Gilkes
In , shortly after Semenza published his seminal work on HIF regulation of EPO expression, collaborated on a paper in the journal Nature Genetics about mutations causing familial polycythemia in the Chuvash, a Turkic ethnic group in western Russia.
By Prchal’s account, he is one of a small handful of scientists Semenza has collaborated with continuously over the years, outside of Semenza’s mentors and mentees.
“I’ve been very fortunate to have had really superb mentors at every step of my career,” Semenza said. “I was really inspired by a school biology teacher; her name was Dr. Rose Nelson, and she started me on the way to my career in science.”
While Semenza encourages independence in his trainees, he continues to collaborate with a number of them. One of his former postdocs, , who studies the hypoxic breast tumor microenvironment, started as an assistant professor at Hopkins in 2015.
“Having a mentor that was both a clinician and a scientist was extremely important and impactful for my training,” Gilkes said. “Gregg helped me to focus on research that could make a true impact on improving outcomes.”
Semenza pointed out early in her postdoctoral fellowship that metastasis, rather than the primary tumor, is what kills patients with breast cancer. Gilkes then focused her research efforts on oxygen’s role as a critical determinant in the metastatic process.
She noted that even with as many as 15 people in the lab, Semenza always responded quickly to her questions.
“I try to do that for my students, because that was really important to me,” she said. “It made me feel like he was very interested in the work that I was doing and that it was important to him. But it’s difficult to emulate.”
Beyond the lab
In addition to their value as basic research, Semenza, Ratcliffe and Kaelin’s discoveries about oxygen sensing have helped pave the way for drugs, now in advanced clinical trials, that could kill cancer cells by starving them of oxygen through inhibiting the prolyl hydroxylases that modify HIF or inhibiting HIF-1 alpha outright. While some have been approved in China, the drugs have yet to be marketed.
“I think in the U.S., we’ll probably have an answer in one to two years from these trials,” Semenza said.
“We’re doing those experiments because we hope that we’re going to be able to do something that’ll impact on public health. We haven’t gotten there yet, so we’ll just keep going.”
In memoriam: Paul Greengard
 As we celebrate Gregg Semenza’s Nobel prize, we look back on laureates Paul Greengard and Sydney Brenner who died earlier this year. A retrospective of Brenner can be found here.
As we celebrate Gregg Semenza’s Nobel prize, we look back on laureates Paul Greengard and Sydney Brenner who died earlier this year. A retrospective of Brenner can be found here.
Neuroscientist Paul Greengard, who won the Nobel Prize in physiology or medicine in 2000 for discovering how brain cells react to dopamine, died April 13 at the age of 93.
Greengard was born in Brooklyn on Dec. 11, 1925. After graduating from high school, he enlisted in the Navy, where he became an electronic technician and was assigned to a team at the Massachusetts Institute of Technology that worked on early-warning radar system to protect American ships in World War II. He then went to Hamilton College, where he majored in physics and mathematics. He received his Ph.D. in biophysics from Johns Hopkins University in 1953, and joined the faculty at Yale University in 1968 after years of postdoctoral work and a stint in the pharmaceutical industry.
At Yale, he conducted his then-underappreciated groundbreaking research on neurochemical signal transduction, which went against the prevailing notions that nerve cells communicated solely through electrical signals.
He started at The Rockefeller University in 1983, where he served as the founding director of the Fisher Center for Research on Alzheimer’s Disease, a topic he researched, along with Parkinson’s disease, depression and schizophrenia, until his death.
Greengard and his wife Ursula von Rydingsvard used the $400,000 Nobel award to found an annual prize at Rockefeller University for outstanding female biomedical scientists. The Pearl Meister Greengard Prize, named after Greengard’s mother, who died giving birth to him, has been awarded since 2004. Greengard is survived by his wife Ursula, sister Linda Greengard, three children and six grandchildren.
Enjoy reading 91��ɫ��ý Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from 91��ɫ��ý Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in People
People highlights or most popular articles

Sketching, scribbling and scicomm
Graduate student Ari Paiz describes how her love of science and art blend to make her an effective science communicator.

Embrace your neurodivergence and flourish in college
This guide offers practical advice on setting yourself up for success — learn how to leverage campus resources, work with professors and embrace your strengths.

Survival tools for a neurodivergent brain in academia
Working in academia is hard, and being neurodivergent makes it harder. Here are a few tools that may help, from a Ph.D. student with ADHD.

Quieting the static: Building inclusive STEM classrooms
Christin Monroe, an assistant professor of chemistry at Landmark College, offers practical tips to help educators make their classrooms more accessible to neurodivergent scientists.

Hidden strengths of an autistic scientist
Navigating the world of scientific research as an autistic scientist comes with unique challenges —microaggressions, communication hurdles and the constant pressure to conform to social norms, postbaccalaureate student Taylor Stolberg writes.

Richard Silverman to speak at 91��ɫ��ý 2025
Richard Silverman and Melissa Moore are the featured speakers at the 91��ɫ��ý annual meeting to be held April 12-15 in Chicago.

